

In November, 2000 the International Conference on Harmonisation (ICH) guideline agreed upon preparation of Common Technical Document (CTD) format for submission of application for registration of medicinal product to the regulatory authority of United States, European Union and Japan.
It helps in harmonization of regulatory documentation among those 3 main regions of the world which makes the drug-registration process much simple and hurdle-free. In September, 2002 CTD was re-edited with numbering and section headers changes and in December, 2002 it was approved by the steering committee for the current version with a minor editorial correction.
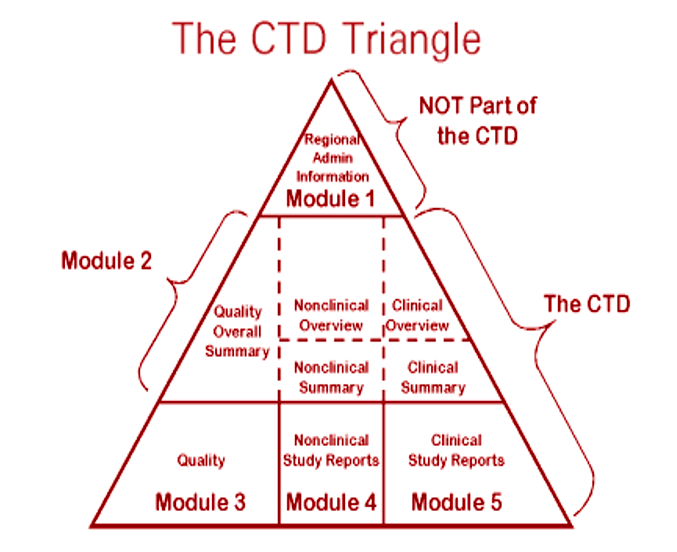
Like many countries the Central Drugs Standard Control Organization (CDSCO), Govt. of India has also adopted the CTD format for registration of the pharmaceuticals. The CTD is organized into 5 modules viz. M1 (regional administrative information), M2 (overall summaries), M3 (quality), M4 (non clinical study report) and M5 (clinical study report).
VICH, also known as International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products is a trilateral programme for the 3 main regions of the world viz. United States, European Union and Japan. The objective of VICH is to develop a common technical requirement for the registration of pharmaceuticals and biologicals intended for veterinary use. It was officially launched in April, 1996.
The dossier for veterinary medicinal products comprises of 4 parts.
Part 1: Summary of the dossierPart 2: Quality
Part 3: Safety & Residue test
Part 4: Efficacy


