The guideline indicates right format for presenting the nonclinical data obtained from a range of studies performed at preclinical level.
However, it does not suggest the requirement of a particular study intended for a specific drug registration.
The Module 4 is comprised of
4.1 Table of contents
4.2 Study reports
4.3 Literature references
The table of contents enlists all kind of nonclinical study reports conducted and provides the location of each report in the CTD
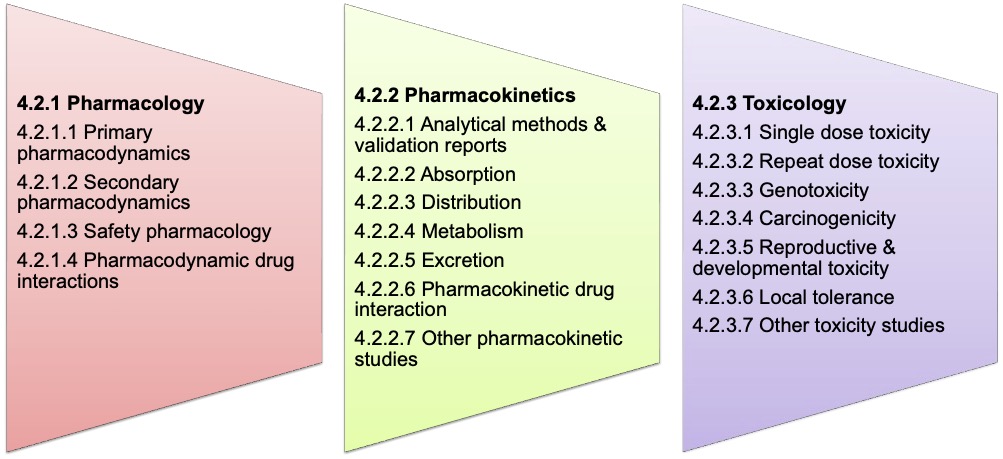
The study report broadly delineates
4.2.1 Pharmacology
4.2.2 Pharmacokinetics
4.2.3 Toxicology